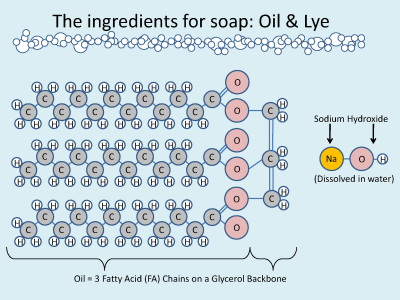
The reason we need soap is that we wash our clothes, dishes, and bodies with water. Most ‘dirt’ is greasy from oils coming off our skin and food. Even if you’ve never studied chemistry you will have heard that oil and water do not mix. Water is a polar molecule which means it has a positively-charged end and a negatively-charged end. When other polar molecules (like alcohol) are put in water, all the positive ends and negative ends are attracted to each other like little magnets and so the two substances mix. Molecules, like oil, that are nonpolar don’t have charged ends. When they are put in water they try to avoid all the little magnets and lump together.
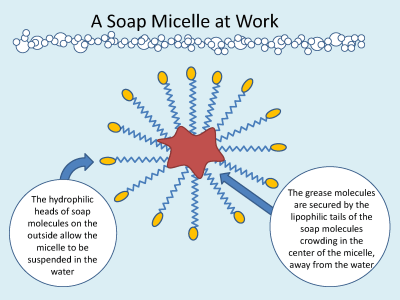
Soap is a special molecule. One end of the soap molecule is charged (polar) while the other end is not charged (nonpolar). What happens when soap is added to water is that the polar end seeks out water and the nonpolar end seeks out other nonpolar molecules like oils. The soap molecules dig their nonpolar tails into the grease while their polar heads are being little magnets with the water molecules, making a ball called a soap micelle. In this way, soap carries grease in water even though the grease itself cannot dissolve in water!
I made this little tutorial to explain it pictorially – let me know if it helps!






nice presentation! :Dvery easy to understand 🙂
These diagrams are amazing! They are perfect for getting these concepts across. Thanks a lot!
absolutely incredible 🙂 this is so much more easier to understand 😀 great job!
Thanks! Used it to explain the concept of soap to gifted 2nd graders in a Kitchen Chemistry course
thank you! very helpful 🙂